A new regulation of plastidial thioredoxins
Adenylates regulate the action of thioredoxins via binding to a CBS domain protein in Arabidopsis plastids.
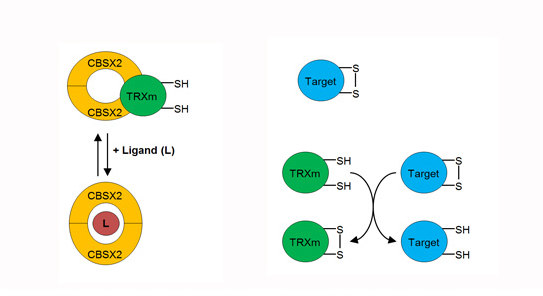
Two teams of IPS2 (CCARS and OGE) recently collaborated and published in Plant Physiology) a study unraveling a new mode of regulation of redox control in the chloroplast, through the interaction between thioredoxins (TRXs) and a cystathionine--synthase protein (CBSX). CBS domains are energy sensors that regulate protein activity through their ability to bind adenosyl ligands. TRXs are disulfide bridge oxidoreductases involved in the redox regulation of numerous enzymatic activities, as well as in the regeneration of thiol-dependent peroxidases. We have shown that CBSX2 specifically inhibits the functions of m-type TRXs. Adenylates (ADP or ATP) prevent this effect by abolishing the direct interaction of CBSX2 with reduced TRXm1/2. Using Arabidopsis T-DNA mutants, in planta analyses allowed us proposing a new regulatory mechanism of TRXs to maintain high energy levels in the chloroplast for an optimal plant growth.
05/09/2022
